В продолжение дискуссии в предыдущих ветках где были опубликованы наблюдения с дивертикулом пищевода и миомой.
КТ является очень хорошим методом визуализации ЖКТ, при условии адекватного наполнения контрастом и применения адекватного протокола съёмки.
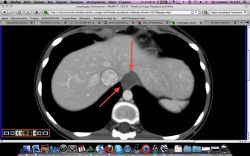
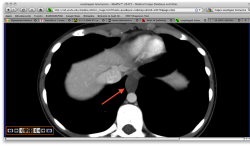
1.Миома пищевода. Уважаемый Петрович, специально для вас, БОЛЬШИЕ картинки с миомой пищевода: изображения с сайта Medpix. http://rad.usuhs.edu/medpix/editors_image.html?mode=quiz&quiz=no&map=&imid=26375&page=#pic
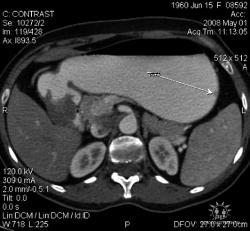
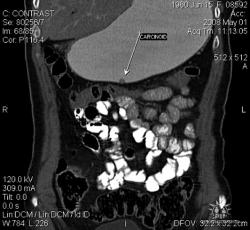
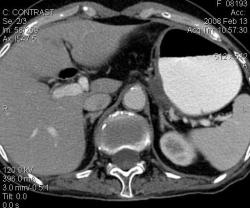
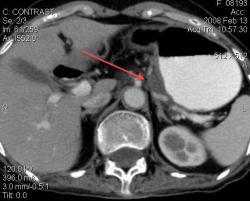
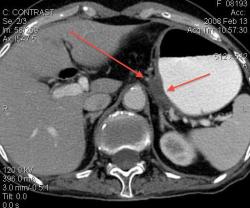
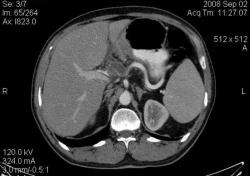
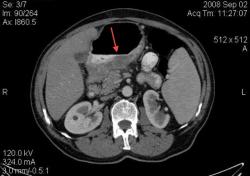
2.Карциноиды желудка. Представленные карциноиды не превышают в размере 4 мм. Удалены под контролем эндоскопии; диагноз верифицирован морфологически. (собственное наблюдение)
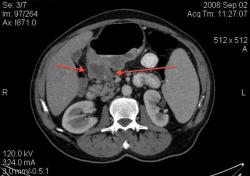
3.Лимфома желудка (MALT Lymphoma). Обратите внимание, экзофитного компонента нет, такие лимфомы растут интра-мурально. Диагноз верифицирован морфологически. (собственное наблюдение).
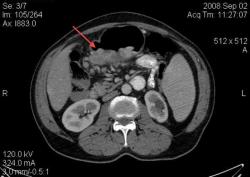
4.Рак желудка. Аналогичные наблюдения я уже демонстрировал ранее. В настоящее время, таких пациентов не оперируют без предварительной КТ визуализации, которая позволяет провести стадирование процесса и адекватное планирование объёма оперативного вмешательства. (собственное наблюдение).
Ну и напоследок, не самая свежая статья из журнала Европейская Радиология за 2006 год; где проведено сравнение КТ-эзофагографии с традиционной рентгеноскопической методикой. Как видите, КТ метод не только не уступает, но в некоторых моментах превосходит классическую рентгеноскопию в вопросах диагностики патологии пищевода.
Спасибо за внимание.
| European Radiology |
| © Springer-Verlag 2006 |
| 10.1007/s00330-006-0337-8 |
Gastrointestinal
Se Hyung Kim1, Jeong Min Lee1, 2  , Joon Koo Han1, 2, Young Hwan Kim3, Jae Young Lee1, Hyun-Ju Lee1, Kyung-Sook Shin1, 4 and Byung Ihn Choi1, 2
, Joon Koo Han1, 2, Young Hwan Kim3, Jae Young Lee1, Hyun-Ju Lee1, Kyung-Sook Shin1, 4 and Byung Ihn Choi1, 2
| (1) | Department of Radiology, Seoul National University College of Medicine, 28, Yongon-dong, Chongno-gu, Seoul, 110-744, South Korea |
| (2) | Institute of Radiation Medicine, Seoul National University Hospital, Seoul, South Korea |
| (3) | Department of Thoracic Surgery, Seoul National University Hospital, Seoul, South Korea |
| (4) | Chungnam National University Hospital, Daejeon, South Korea |
 | Jeong Min Lee Email: leejm@radcom.snu.ac.kr |
Received: 27 October 2005 Revised: 24 April 2006 Accepted: 9 May 2006 Published online: 15 June 2006
Keywords Esophagus, CT - Esophagus, endoscopy - Esophagus, barium study - Esophagus, neoplasms
Esophageal cancer and leiomyoma are two of the most common esophageal neoplasms. Esophageal cancer is responsible for 4% of all newly diagnosed malignant gastrointestinal neoplasms and is the sixth leading worldwide cause of cancer death [1]. As patients with esophageal neoplasms usually present with dysphagia, the initial evaluation includes esophagogastroduodenoscopy or a barium swallow study. The preoperative work-up and staging algorithms for esophageal tumors are evolving as more accurate modalities for preoperative staging become widely available [2]. According to the National Comprehensive Cancer Network guideline for the staging of patients with suspected esophageal cancer, computed tomography (CT) and endoscopic ultrasonography (EUS) are mainframe in the staging scheme, while barium swallow study, bronchoscopy, laparoscopy, and positron emission tomography (PET) or PET-CT are optional [3].
With the recent advances in multidetctor CT (MDCT) and in the three-dimensional (3D) imaging technique, combined interpretation of 2D axial, multiplanar reconstruction (MPR) and 3D-rendered images, may provide enhanced diagnostic capability to stage cancers in various organs [4–7]. In some institutions, however, barium study is still incorporated into the routine preoperative workup of patients with esophageal tumors as it provides intuitive information regarding the longitudinal extent of tumor involvement, which might be helpful for surgical or radiation therapy planning. Based on our experience with virtual endoscopy of the stomach using MDCT [8], we assumed that MDCT imaging with 3D CT esophagography (CTE), including CT endoscopy, might provide an intuitive and comprehensive evaluation of esophageal tumors; i.e., locoregional staging, endoluminal morphology, longitudinal extent, degree of luminal stenosis, and invasion to adjacent mediastinal structures. Until recently, however, there has only been few English reports on the diagnostic capability of MDCT to evaluate esophageal lesions [9, 10].
Therefore, the purpose of our study is to determine the diagnostic usefulness of MDCT imaging with 3D CTE for the evaluation of esophageal tumors.
From August 2004 to February 2005, 23 consecutive patients (20 men and three women; age range, 43–86 years; mean age, 60.8 years) with suspected or known esophageal neoplasms, were referred from the Internal Medicine or Thoracic Surgery Department of our tertiary center for participation in this study. The study was approved by our Institutional Review Board, and written informed consent was obtained from each patient.
We used a 16-detector-row CT scanner (Sensation 16; Siemens Medical Systems, Forchheim, Germany) for CTE. Before CT examination, 10 mg of butyl scopolamine were administered intravenously in order to facilitate hypotonia and minimize peristalsis. For air insufflations, a 16-F end-hole Foley catheter connected to a mechanical inflator placed beside the CT console using a long plastic connector, was inserted into the upper esophagus through the mouth. Air injection was done by one radiologist from 12 s before scanning to the time the scanner passed the gastroesophageal junction at a rate of 700 ml (cc)/30 s. The total amount of injected air was recorded.
Two sessions of CT scanning were performed in the arterial and portal venous phases. Two scanning passes were performed through the entire thorax to mid-abdomen with 16×0.75 mm detector configuration, 1 mm section thickness, table feed of 12 mm/rot, 0.5 s rotation time, and 160 mAs/120 kVp. Images were reconstructed at an interval of 0.7 mm to obtain a high-quality dataset for CT esophagographic reconstruction, and the thin-section dataset was sent to a PC installed with dedicated 3D software (Rapidia, INFINITT). Each scanning pass required an acquisition time of about 15 s. The scanning delay was 10 s after reaching enhancement of the descending aorta up to 100 HU, as measured by a bolus-tracking technique [11] for the arterial phase. After completing the arterial phase scanning, a 20-s inter-scan delay was used for the portal phase. Therefore, the portal phase scanning began approximately 68 s after the initiation of contrast injection. A 120-ml volume of nonionic contrast material with a concentration of 370 mg I/ml, was intravenously administered using a power injector at a rate of 3–4 ml/s.
After loading the dataset into memory with Rapidia, the images were reconstructed by one of two experienced 3D technologists using a volume rendering technique in a surface-shaded and transparent mode similar to the images obtained in single- and double-contrast barium studies, respectively, and were sent to a picture archiving communication system (PACS). In the endoscopic image, navigation within the esophageal lumen was accomplished by creating a flight path semiautomatically in a cephalad to caudad direction. Virtual endoscopic images were also sent to PACS. Processing of the reformatted images required approximately 10 min per case.
All patients, except for three, underwent a double contrast barium study. Even though barium study is still incorporated into the routine preoperative work-up of patients with esophageal tumors at our institute, it is optional rather than mandatory. In three of our subjects, the clinicians did not order the patients to undergo barium study because the esophageal tumor was too extensive (5 cm, 8 cm, and 16 cm in long diameter, each) and the clinicians judged that the information of esophagoscopy and CT esophagography (surface shaded and transparent modes) was enough to perform palliative chemoradiation therapy. For barium study, a standard procedure was used. The time between barium study and CTE ranged from 0 to 81 days (average 9.8 days); 15 (75%) of 20 patients underwent both examinations within 1 week. Conventional endoscopy was carried out in all patients. No intravenous sedation was induced in any patient. The time between endoscopy and CTE ranged from 0 to 83 days (average 9.8 days); 17 (74%) of the 23 study patients underwent both examinations within 1 week.
The degree of esophageal distension on the surface-shaded and transparent 3D CTE images was evaluated by two reviewers in consensus. The degree of distension in the three esophageal segments was graded using a three-point scale: 1, for good distension; 2, for fair; 3, for poor [12].
MDCT images with 3D CT esophagography including CT endoscopy were independently assessed by two other radiologists, who were blinded to the findings of the conventional studies and the histopathology. For localization, the esophagus was divided into upper, middle, and lower segments. Both radiologists were asked three questions. Firstly, they were requested to note their degree of confidence that a lesion was present in each esophageal segment of each patient, on a five-point confidence scale (1, definitely absent; 2, probably absent; 3, possibly present; 4, probably present; 5, definitely present). Secondly, for lesion characterization, they were also requested to assess their degree of confidence regarding whether each lesion was mucosal (esophageal cancer) or submucosal tumor, on a five-point scale (1, definitely submucosal tumor; 2, probably submucosal tumor; 3, possibly esophageal cancer; 4, probably esophageal cancer; 5, definitely esophageal cancer). Lastly, the radiologists were also requested to determine the tumor (T) and node (N) staging for seven cancer patients who underwent curative esophageal resection. For staging purposes, regional lymph nodes (LNs) were classified as follows: N0, no regional LN metastases; N1, regional LN metastasis. Lymph node metastasis exceeding regional LN was considered to be distant metastasis.
A subjective evaluation of CT esophagography was used to analyze the presence of the lesion and to characterize the lesion at the time of interpretation. Two radiologists were asked to provide a diagnosis according to previously published, established criteria [9, 13–15]. For the determination of the presence of esophageal lesions, the diagnostic criteria we used were as follows. Lesions presenting a thick wall (more than 3 mm in thickness) or mass, with higher or heterogeneous enhancement compared with the adjacent mucosa, obliteration of layered pattern of the esophageal wall, periesophageal fatty infiltration or mass effect to the adjacent structure, and mucosal irregularity or bulging on CT esophagoscopy were considered to be esophageal tumor. For the characterization of esophageal tumor, eccentrically located lesions having a smooth or lobulated margin and intact overlying mucosa and without peritumoral invasion or adjacent fat obliteration were considered to be submucosal tumors.
Additionally, to analyze how well the 3D CTE images correlated with those of the conventional barium study and endoscopy in terms of the endoluminal shapes, luminal narrowing, and the longitudinal extent of the lesions, a direct comparison between the results of the conventional studies and those of the 3D CTE was made by the two reviewers in consensus using a four-point scale: 0, lesion not found; 1, lesion found, but the amount of information on 3D CTE is inferior to that obtained by conventional study; 2, information similar to that obtained by conventional study; 3, information superior to that obtained by conventional study.
The diagnostic performance of MDCT esophagography with respect to lesion detection and characterization by two radiologists, was evaluated using areas (Az) under the receiver operating characteristic (ROC) curve. The univariate z-score test was used to assess the significance of differences in the area (Az) under the two ROC curves. In addition, the sensitivity and specificity of CTE for detecting the lesion and for differentiating a mucosal lesion (esophageal cancer) from submucosal tumor, were determined using the number of patients whose images were assigned a score of 3 or larger and a score of 1 or 2, respectively. Sensitivity and specificity are presented with a 95% confidence interval.
SPSS version 11.0 (SPSS, Chicago, Ill.) software and GraphPad InStat version 3.0 (GraphPad Software, San Diego, Calif.) were used for statistical analysis. For all tests, a P value less than 0.05 was considered to indicate a statistical significance.
On pathologic evaluation, the lesions of 15 study patients were squamous cell carcinomas, six were leiomyomas, and the lesions in the remaining two patients were adenocarcinoma and leiomyosarcoma, respectively. Surgery was performed on 12 patients (mass enucleation in five and Ivor Lewis operation in seven), including five of six with leiomyoma and seven of 16 with esophageal cancer. In the remaining 11 patients, the final diagnosis was proved by endoscopic biopsy.
The size of the lesions ranged from 1 to 15 cm (mean, 5.4 cm). The lesion was located on the upper esophagus in ten patients, middle in six, and lower esophagus in seven. On histopathologic examination of the seven patients who underwent surgery for esophageal cancer, three were staged as pT1, two as pT2, and two as pT3. For the node stage, five cases were staged as pN0 and two as pN1. In one patient, liver and lung metastases were diagnosed with clinical follow-up.
| Esophageal segment | Good | Fair | Poor | P value |
|---|---|---|---|---|
Upper | 21/23 (91%) | 1/23 (4%) | 1/23 (4%) | >0.05 |
Middle | 19/23 (86%) | 1/23 (4%) | 3/23 (13%) | |
Lower | 19/23 (86%) | 2/23 (9%) | 2/23 (9%) |
| Radiologist | Az | Sensitivity (%) | Specificity (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| 95% CI |
| 95% CI |
| 95% CI | ||||
| Lower | Upper | Lower | Upper | Lower | Upper | ||||
Radiologist 1 | 0.954 | 0.874 | 0.989 | 91.3 (21/23) | 72.0 | 98.9 | 95.7 (44/46) | 85.2 | 99.5 |
Radiologist 2 | 0.957 | 0.878 | 0.990 | 91.3 (21/23) | 72.0 | 98.9 | 100 (46/46) | 92.3 | 100 |
P value | 0.936a | 1.000b | 0.495b | ||||||
| Radiologist | Az | Sensitivity (%) | Specificity (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| 95% CI |
| 95% CI |
| 95% CI | ||||
| Lower | Upper | Lower | Upper | Lower | Upper | ||||
Radiologist 1 | 0.954 | 0.764 | 0.992 | 92.9 (13/14) | 66.1 | 99.8 | 100 (7/7) | 59.0 | 100 |
Radiologist 2 | 0.995 | 0.828 | 1.000 | 92.9 (13/14) | 66.1 | 99.8 | 100 (7/7) | 59.0 | 100 |
P value | 0.259a | 1.000b | 1.000b | ||||||
|
| Histopathologic T and N stage | ||
|---|---|---|---|
CTE T stage | pT1 | pT2 | pT3 |
TX | 2 | 0 | 0 |
T1 | 1 | 0 | 0 |
T2 | 0 | 1 | 1 |
T3 | 0 | 1 | 1 |
T4 | 0 | 0 | 0 |
CTE N stage | pN0 | pN1 | M |
N0 | 5 | 0 | 0 |
N1 | 0 | 1 | 0 |
M | 0 | 1 | 0 |
|
| Surface-shaded and transparent VR imagesa | Virtual esophagoscopyb | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | |
Esophageal cancer | 2 | 2 | 9 | 0 | 2 | 4 | 10 | 0 |
Submucosal tumor | 0 | 0 | 6 | 1 | 0 | 0 | 7 | 0 |
P valuec | 0.278 | 0.227 | ||||||
The present study shows that MDCT imaging including CTE can provide comprehensive information regarding the detection and characterization of esophageal tumors, as well as demonstrating endoluminal morphology and the longitudinal extent of lesions. In the present study, the radiologists’ performance for detecting and characterizating esophageal tumors in terms of Az was fairly good. In terms of the detection of the esophageal lesions, the results of our study are markedly superior to those of previous studies (67% sensitivity for tumor detection) [16]. We though that it might be related to a usage of both state-of-the-art MDCT with CTE and meticulous esophageal distension. Indeed, based on our clinical experience, it is quite common that CT fails to demonstrate T1 or T2 esophageal cancer if the above techniques are not used. However, given that LN metastasis from esophageal cancer is close by the location of the primary cancer, the detection and localization of esophageal cancer may encourage radiologists to draw attention to regional LNs.
In the present study, we obtained esophageal distension through air insufflation using intubation and a mechanical inflator. Therefore, most of the esophageal segments were well distended, and the resulting 3D images were adequate for esophageal evaluation. As in CT colonography, an adequate distension of the esophagus is essential for creating high-quality, 3D-rendered images in CTE. However, in previous CTE studies [9, 10], simply taking an effervescent agent can be used to obtain esophageal dilatation. It is anticipated that simply taking an effervescent agent will further increase patient compliance, but, as the authors mentioned in their report, the technique required considerable patient cooperation and proper timing for scanning in order to achieve optimal distension of the esophagus [9]. Based on our experience, when an effervescent agent is simply used, good CTE images cannot be obtained without highly cooperative patients or without effort to proper timing for scanning.
Several imaging techniques, including barium studies, endoscopy, CT, EUS, and PET, are available for use in the evaluation of esophageal tumors. In the literature, the role of CT in the diagnostic work-up of esophageal tumors, is mainly related to the staging. Several characteristics inherent to CT imaging enhance the attractiveness of 3D images. Although information regarding the extraluminal extent, as well as the endoluminal morphology of the esophageal lesions, is critical in the evaluation of esophageal tumors, this kind of information is not available if only axial CT images are obtained. However, according to our study results, MDCT may contribute by adding the advantage of more intuitive and comprehensive imaging to conventional CT. For example, 3D MDCT imaging could provide information regarding endoluminal morphology and the longitudinal extent of esophageal tumors which could be obtained only with conventional barium study until now. In 16 of the 20 tumors in the present study, 3D-rendered CTE images in the surface-shaded or transparent mode, gave similar or superior information to that of barium study regarding the morphology and longitudinal extent of esophageal tumor. In 17 of 23 patients, virtual endoscopic images gave similar information to conventional endoscopy. Particularly, in one submucosal tumor, CTE in the surface-shaded and transparent mode, gave a correct diagnosis of submucosal tumor that barium study could not. Although the length of the tumor is no longer used to define T staging, the exact localization and confining of esophageal tumor in a longitudinal direction, are still important for both classification of regional lymph nodes for esophageal cancer and for planning surgery or radiation therapy, as the classification of regional lymph nodes in esophageal cancer depends on the location of the primary tumor.
Even though we used a thin-section (0.7-mm) dataset and dedicated 3D volume-rendering techniques, fine mucosal changes in two patients with superficial (1 and 8 mm in depth) esophageal cancer could not be detected on the CTE images. However, comprehensive and retrospective review of the CTE images along with the barium study and endoscopy results demonstrated those subtle mucosal changes. Other causes that made CTE inferior to conventional studies were inadequate distension of the involved esophagus and its intrinsic limitation of not depicting qualitative information such as easy touch bleeding.
Recent developments of MDCT allowed the production of high quality MPR images, and several previous publications have demonstrated that combined interpretation of 2D axial and MPR images may provide enhanced diagnostic capability to stage cancers in various organs including the colon, pancreas, biliary tree, and stomach [4–7]. However, in our study, the overall accuracy of CTE with MPR images regarding tumor and node staging, was 42.9% and 85.7%, respectively, while conventional CT has been reported to have a 54% accuracy for the T staging and a 66–83% accuracy for the N staging [16–19]. Although our results cannot be generalized as we analyzed the findings of only a small number of cases which were proven at surgery, MDCT still has intrinsic limitations regarding local staging. CT cannot effectively differentiate paraesophageal nontumoral infiltration from tumor infiltration into adventitia and cannot exactly determine adjacent organ invasion. We speculated that the scanty fat between the esophagus and adjacent structure may limit the exact application of “obliteration of the fat plane between the esophagus and adjacent organs” which is the CT sign suggesting adjacent organ invasion. For the N stage, CT cannot differentiate benign causes of enlargement from metastatic tumor. In addition, enlarged lymph nodes adjacent to an esophageal cancer may not be detected because they are inseparable from the primary lesion. Even though we take them into consideration in our study, CT performance for local staging is discouraging. It can be responsible for a small number of cases (n=7) and for selection bias because we included only patients who underwent surgery in order to determine T and N staging.
There are several limitations to our study. Firstly, our study sample was relatively small; in particular, surgery provided confirmation regarding tumor and node staging in only a subset of all patients studied. However, our preliminary results demonstrate the potential feasibility of CTE to detect and characterize esophageal tumors as well as to provide information which can be obtained only from conventional studies. Our experience particularly highlights the feasibility of CTE as a comprehensive imaging modality for the evaluation of esophageal tumor. Secondly, as we used intubation for esophageal distension, it required that the radiologist expend additional effort and worsened patients acceptance compared with simple taking an effervescent agent. Therefore, an effort to improve patient compliance should be exerted. If a more effective effervescent agent or scanning techniques can be developed, a further increase in patient compliance could be achieved in CTE without intubation.
In conclusion, a comprehensive 3D MDCT examination including CTE has the potential to serve as a comprehensive imaging modality for evaluating esophageal tumors as it provides accurate information regarding the detection and characterization of esophageal tumors, as well as the similar information as that which would be provided only by conventional studies.


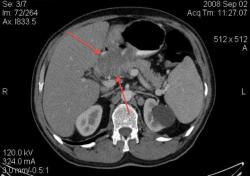
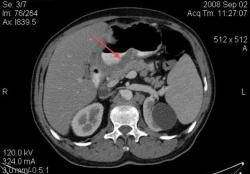
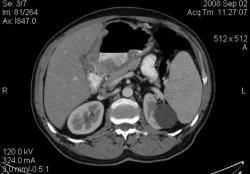
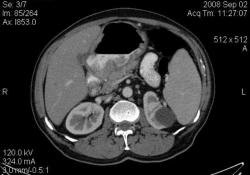




Большое спасибо, Уважаемый Dr.Mario! Публикация очень интересная! Если можно ещё вопросы.
Карциноиды относятся к редко встречающимся злокачественным опухолям эпителиального происхождения, при таких размерах отличить их от нередко встречающихся полипов рентгенологически невозможно. А при КТ-исследовании какое заключение Вы дали? Или предшествовала эндоскопия с её возможностью биопсии?
Это специально для поиска мелких опухолей так растянут желудок (чтобы складки не мешали)? Что это за контраст использовали (явно не бариевая взвесь, или же сильно разбавленная)?
Неоднозначно всё
По поводу бария; я уже писал Вам в другой ветке http://www.radiomed.ru/cases/leyomioma. Он не используется при КТ.
Желудок растянули уже дополнительно; я стандартно даю на столе выпивать 2-3 стакана контраста сразу перед исследованием; этого в большинстве случаев достаточно. У данной пациентки, на изначальных сканах (к сожалению я их не сохранил, давно было дело), возникло подозрение на локальное утолщение стенки, поэтому дал выпить на столе дополнительно ещё контраст, потом сделал контрольные сканы с хорошо растянутым желудком. Эндоскопия с удалением карциноидов была уже после КТ. Кстати, жалобы пациентки не были связаны с патологией желудка; это как бы "случайная находка". По поводу заключения, насколько я помню, я написал экзофитные опухоли желудка, карциноиды были перечислены в диф. ряду, наряду с полипами и метастазами меланомы.
Let me see...
radiographia.ru
С Раком желудка картина ясная: когда он большой, да ещё и экзофитный — лёгкая добыча рентгенолога. Но вы, конечно, можете видеть прямые признаки прорастания в соседние органы и метастазирования
Интрамурально растущая Лимфома желудка, наоборот рентгенологическим методом выявить очень проблематично (тем более в обл. свода желудка). И эндоскопия здесь не поможет.
Но вот вопрос, Dr.Mario: диагностируете ли Вы гастриты? Рентгенологу для этого нужно увидеть тонкий рельеф (микрорельеф) слизистой желудка.
Неоднозначно всё
Я специально привел пример лимфомы, что бы было понятно важная роль именно КТ метода, он в принципе остаётся единственно рабочим при такой патологии. Насчёт гастрита-это вообще не наша тема (радиологов); для этого существует специализация "эндоскопист", которые обязаны при этом брать биопсию слизистых и проводить анализ на H. Pylori. Не обижайтесь; но если кто нибудь в Штатах, Европе или даже у нас, пошлёт пациента на рентгеноскопию желудка с направлением: гастрит? То радиолог просто покрутит пальцем у виска и никогда не возьмёт пациента на скопию, а просто направить к эндоскописту... За всё свое время обучения специализации (около 5 лет), направления на рентгеноскопию с диагнозом "гастрит", я помню только когда находился в СНГ.
Let me see...
radiographia.ru
А ещё вопрос можно? Относительно миомы пищевода.
По представленным сканам (или другим, лучшего качества) можно решить, что опухоль находится в подслизистом слое и на слизистую не выходит?
При рентгеноскопии это обычно не проблема, поскольку видим рельеф слизистой и перистальтику. А можно и раздуть.
Неоднозначно всё
Конечно можно, просто надо попросить пациента глотнуть контраст во время съёмки (у себя в отделения я применяю такую методику), а можно и раздуть. Различные методики описаны в приведённой статье мной статье по КТ эзофагографии. Кстати, очень неплохой и достаточно информативный метод, который сейчас осваивают наши эндоскописты-УЗИ при эндоскопии.
Let me see...
radiographia.ru
Ещё раз большое спасибо, Уважаемый Dr.Mario, за интересную публикцию. Статью я прочитаю обязательно. Не сразу. На перевод потребуется время.
Неоднозначно всё