Больная, 26 лет: БОМЖ 6 лет, наркомания 10 лет, ВИЧ, ХПН, цирроз печени, асцит, спленомегалия...; стрептодермия? рожистое воспаление обеих стоп? Сознание ясное, состояние тяжелое, самостоятельно не передвигается (отек нижних конечностей). Что думаете коллеги?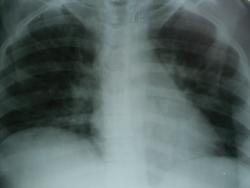
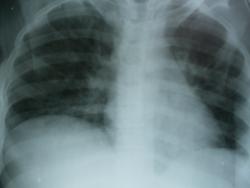
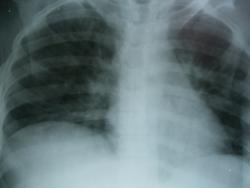
качество изображения не позволяет судить о чем либо
да, мутновато. но все же.
Уважаемый serega! Вы озаглавили свою публикацию:
саркома КапошиВы на самом деле полагаете, что на основании выставленных Вами рентгенограмм грудной полости можно заподозрить именно эту нозологию?
Let me see...
radiographia.ru
нет, я так не пологаю. Был еще снимок в динамике ч/з неделю ( но он к сожалению утерян)- там появились разноколиберные очаги, я написал септическую эмболию. на вскрытии саркома, поздняя стадия. если знаете, какие специфические рентген. симптомы для данной саркомы существуют?
Материала по теме достаточно. Предлагаю ознакомистаы сначала с общими положениями по Саркоме Капоши, а в следующей статье Вы найдёте более менее внятное описание радиологических признаков.
Primary Surgery: Volume One:
Chapter 18. Primary oncology
Kaposi's sarcoma
Kaposi's sarcoma is an angiosarcoma consisting of multiple endothelial channels surrounded by spindle cells. There are four types. The second two are related to HIV.
Classical Kaposi's sarcoma (rare) affects elderly men in the industrial world. It has a variable behaviour, and is often indolent, but not always so.
Endemic Kaposi's sarcoma (KS) has been recognized in equatorial Africa since at least the 1940's. It has an adult and a paediatric form, and is much more common in males (10:1). The patient is typically a male labourer of about 40, who has had symptoms for 18 months before the growth of his lesions forces him to seek treatment.
His symptoms start with oedema of his leg(s), usually at the end of the day, which is gone by the morning. At first his oedema is intermittent; later, it becomes more constant, and eventually ceases to pit on pressure. Once locally progressive disease has developed, the normal temperature gradient of his affected limb is reversed, so that it is warmer peripherally than it is proximally.
The indolent form (common) occurs after a variable period of oedema, as 3 to 5 mm nodules which are more easily felt than seen, and are the typical lesions of endemic Kaposi's sarcoma. They form in or under the skin of his feet and legs and less often his hands and lower arms, usually in more than one limb, and on his right arm more often than his left. Involvement is usually asymmetrical, and one limb may be normal. The nodules are usually the same colour as the surrounding skin, but may be purple. He may also have larger subcutaneous nodules along his superficial vessels. Nodules may persist unchanged for years, and disappear spontaneously, only to reappear at new sites. Traditional practitioners sometimes excise them, usually with good results. Less commonly, he has asymmetrically distributed plaques, millimetres to centimetres in diameter, starting on the sides and dorsum of his feet, and later on his palms and soles. These have a palpable edge and are darker than the surrounding skin.
The nodes at the root of his most diseased limbs are often enlarged, but he has no generalized lymph node enlargement. He loses some weight and becomes moderately anaemic. After several years he may have pleural effusions (see below), but is unlikely to have more than an occasional lesion in unusual sites (his conjunctiva, mouth, head, neck, or trunk).
The infiltrating form (common) appears in a previously oedematous limb as a diffuse, ill-defined, dark thickening of his skin and deeper tissues, with some hyperkeratosis. It is a hot ''woody' infiltration which creeps proximally to cause joint deformity, stiffness, and disability. This infiltration is more widespread than the plaques described above, and has no raised edge. It may occur alone, but is usually accompanied by nodules, or aggressively growing tumours. The bones of his infiltrated feet or hands may show periarticular osteoporosis, followed by multiple erosions, which may enlarge until his phalanges and metatarsals can no longer be seen on an X-ray. No new bone is formed, except in response to successful treatment.
The locally aggressive form (fairly common) presents as one or more rapidly growing, cauliflower-like tumours, anywhere on his feet, legs, or hands; they arise from existing nodules, which may have been unchanged for months or years. These aggressive lesions ulcerate, and discharge quantities of offensive fluid with a characteristic smell. He has other unchanged nodules proximal to the tumour and around it, and there is always the infiltration described above, somewhere in the same or another limb. If he presents earlier, you may see one of these aggressive lesions before it has started to ulcerate.
The lymphadenopathic form (uncommon) presents in an African child or adolescent as symmetrically enlarged, slightly irregular, fleshy, firm (but not hard), nodes in all sites. He may also have a few atypical skin lesions.
There are two HIV-related varieties of Kaposi's sarcoma:
HIV-related Kaposi's sarcoma of Caucasians in the industrial world. This differs somewhat from that in sub- Saharan Africa.
Atypical African Kaposi's sarcoma (AAKS) was first recognized in the early 1980s. More than 90% of patients are HIV positive, and the sex ratio is more nearly equal (males to females 5:1). At presentation men have a mean age of 35 and women of 25. The patient is likely to have had symptoms for about 8 months, which include loss of weight, and almost always a completely symmetrical generalized lymph node enlargement. You can usually find skin lesions somewhere. Look for irregular 3 to 30 mm easily palpable dark plaques, which are more common on his face, head, neck, trunk, and proximally on his limbs. You may also find them in his throat, or anywhere in his mouth (50% of cases), where they are most often seen on his palate.
The lesions on his mucosa start as a flat purple stain, which becomes raised and then nodular, before it finally ulcerates, sometimes covered by a layer of thrush. Occasionally, his entire gums are infiltrated. Sometimes, he has nodules, particularly on his insteps, and on his thighs along the line of his long saphenous veins, where there may be oedema and infiltration. He may also have oedema of his extremities, which can be severe and resemble the endemic form of the disease, but unlike the latter, it may also involve his trunk, head, and neck. There is a 5% chance that he will have no skin or mucosal changes, and if so, his prognosis seems to be better. He usually has at least one opportunist infection. In spite of all this, he may respond to chemotherapy, and return to work for months, or even years, so it is well worth giving.
Bayley Anne C, ''Atypical African Kaposi's Sarcoma'. Report to the conference ''AIDS in Africa; Naples 1987'. In press. Karger, Basle.
KAPOSI'S SARCOMA ENDEMIC AFRICAN KAPOSI'S SARCOMA DIAGNOSING ENDEMIC KAPOSI'S SARCOMA. Look carefully for typical nodules, which you will almost certainly find somewhere. Feel for a reversed temperature gradient, especially in the patient's legs (see above).
If he has multiple nodules, and mycetoma is rare, biopsy is unnecessary, because the lesions are so typical.
THE DIFFERENTIAL DIAGNOSIS of endemic Kaposi's sarcoma includes leprosy (30.5), fungal infections of his feet with brawny oedema, and chronic osteitis (7.13) of his feet and legs (sometimes secondary to ulceration in leprosy, 30.5), tropical ulcer (31.2), mycetoma (31.3), the hyperkeratosis of lymphedema (31.4), and onchocerciasis.
PROGNOSIS UNTREATED. The indolent type: lesions grow slowly and cause little morbidity. The aggressive type: lesions grow steadily and lead to an early death; the superficial ones ulcerate and cause considerable distress. In the lymphadenopathic type lesions also grow steadily.
MANAGEMENT. [f41]If he has the indolent form of the disease, he needs no treatment. Follow him up carefully, because other types may develop.
If he has any other form of the disease, he needs chemotherapy and, rarely, surgery.
AAKS [s7]ATYPICAL AFRICAN KAPOSI'S SARCOMA DIAGNOSIS. If he has visible masses of symmetrical nodes, each 3[nd]4 cm, AAKS is likely, so search his skin and mouth carefully for characteristic lesions, and if necessary biopsy one.
BIOPSY. is unnecessary if: (1) The diagnosis is obvious clinically. (2) He has symmetrically enlarged nodes [lt]2 cm, because it will probably only show the reactive hyperplasia of HIV infection.
Biopsy is useful because it may reveal a treatable condition if: (1) He has symmetrical nodes (5%) [mt]2 cm without the characteristic skin or mucosal lesions which allow you to diagnose AAKS clinically. (2) He has large asymmetric nodes (e.g. large nodes one side of his neck, and small ones, or none, on the other). If so, expect to find tuberculosis or lymphoma.
PROGNOSIS AND STAGING. In Lusaka a staging scheme has been proposed for prospective testing based on about 500 patients followed for 2 years.
Stage One. Lymphadenopathy alone (diagnosis proved by biopsy). No oral or skin lesions. A normal chest X-ray. Little or no weight loss.
Stage Two. Any combination of lymph node enlargement, skin lesions, and oral disease, without oedema of his head, neck, trunk, or bikini area. No CNS signs. A normal chest X-ray, and only moderate weight loss.
Stage Three. As for Stage Two, but with any of the following: gross weight loss, cough, dyspnoea, bilateral basal infiltration or pleural effusions, neurological symptoms (without evidence of cryptococcal or tuberculous meningitis at lumbar puncture), oedema of the trunk, face or bikini area.
Imaging Features of Pulmonary Kaposi Sarcoma–Associated Immune Reconstitution Syndrome
Myrna C. B. Godoy1,2, Hannah Rouse1, Jacqueline A. Brown1, Peter Phillips3, David M. Forrest4 and Nestor L. Müller5
1 Department of Radiology, St. Paul's Hospital, University of British Columbia, Vancouver, BC, Canada.
2 Department of Radiology, New York University School of Medicine, 650 First Ave., 600-A, New York, NY 10016.
3 Division of Infectious Diseases, St. Paul's Hospital, University of British Columbia, Vancouver, BC, Canada.
4 Division of Infectious Diseases, Nanaimo Regional Hospital, Nanaimo, BC, Canada.
5 Department of Radiology, Vancouver General Hospital, University of British Columbia, Vancouver, BC, Canada.
Received January 29, 2007; accepted after revision May 12, 2007.
Abstract
OBJECTIVE. The purpose of this study was to analyze the radiologic features of pulmonary Kaposi sarcoma–associated immune reconstitution syndrome. The syndrome is a phenomenon characterized by clinical deterioration of the condition of HIV-positive patients after initiation of highly active antiretroviral therapy.
MATERIALS AND METHODS. The study included four patients at our institution who fulfilled the diagnostic criteria for pulmonary Kaposi sarcoma–associated immune reconstitution syndrome from 2001 to 2006. All patients were men (mean age, 43 years; range, 31–59 years). Images reviewed included chest radiographs obtained before highly active antiretroviral therapy, radiographs and chest CT scans obtained at appearance of the symptoms of Kaposi sarcoma–associated immune reconstitution syndrome, and follow-up radiographs and chest CT scans during immune reconstitution syndrome.
RESULTS. The radiographic findings of Kaposi sarcoma–associated immune reconstitution syndrome included reticular and reticulonodular opacities (n = 4), areas of consolidation (n = 3), septal lines (n = 3), and pleural effusion (n = 3). The CT findings in all four patients were ill-defined pulmonary nodules and interlobular septal thickening. Three of the patients had a CT halo sign, areas of consolidation, ground-glass opacities, lymphadenopathy, and pleural effusion. The areas of consolidation in three subjects who did not receive chemotherapy increased markedly after 14–20 days. CT performed during the initial symptoms of immune reconstitution syndrome in these three subjects showed less than 5% parenchymal involvement. Follow-up CT showed 26–50% involvement in two patients and more than 50% involvement in one patient.
CONCLUSION. The radiologic findings of pulmonary Kaposi sarcoma–associated immune reconstitution syndrome are similar to the findings described in patients with Kaposi sarcoma without the syndrome, but the extent of abnormalities tends to increase with the development of the syndrome.
Keywords: AIDS • cancer • CT • Kaposi sarcoma • lung disease
Introduction
Since the introduction of highly active antiretroviral therapy (HAART), there has been a significant decrease in AIDS-related morbidity and mortality as a result of suppression of HIV replication and immune reconstitution in most treated patients with HIV infection [1, 2]. Despite the undeniable benefits of HAART in preventing opportunistic infections and malignant disease, these conditions may temporarily worsen when antiretroviral therapy is started, a clinical response known as immune reconstitution syndrome [3–8]. This syndrome is an inflammatory reaction against microbiologic pathogens and antigens that occurs soon after initiation of HAART and is characterized by a deterioration in clinical condition despite improvement in the HIV RNA level and, usually, by an increase in CD4 cell count [3–8]. Immune reconstitution syndrome can occur during or soon after a patient is treated for an opportunistic infection or as a new clinical manifestation of a previously subclinical infection [3, 9, 10]. The pathogenesis of immune reconstitution syndrome is still unclear but probably involves HAART-induced restoration of pathogen-specific immune responses against infectious and noninfectious antigens [3, 4, 7–11]. Other names for this syndrome are immune restoration syndrome, immune reconstitution inflammatory syndrome, and immune reconstitution phenomenon [3–5].
Immune reconstitution syndrome has been characterized in a variety of HIV-related opportunistic diseases, including Mycobacterium avium complex infection, Mycobacterium tuberculosis infection, cryptococcosis, cytomegalovirus infection, Pneumocystis jiroveci pneumonia, herpes zoster, and progressive multifocal leukoencephalopathy [4–6, 10, 11]. It is estimated that 10–25% of patients who start HAART experience immune reconstitution syndrome [10].
HAART is a key component in the management of Kaposi sarcoma (KS) in patients with HIV infection and often leads to stabilization and regression of KS lesions [12–15]. Occasional reports [4, 6–8, 16–22], however, describe marked clinical deterioration after initiation of HAART, the signs and symptoms being consistent with KS-associated immune reconstitution syndrome. The aim of our study was to analyze the radiographic and CT findings of pulmonary KS-associated immune reconstitution syndrome.
Materials and Methods
The study included four patients consecutively registered at our institution from 2001 to 2006 and fulfilling the diagnostic criteria for pulmonary KS-associated immune reconstitution syndrome (Table 1). All patients were men 31–59 years old (mean age, 43 years). The diagnosis was established by the presence of the following criteria: biopsy-proven KS in skin, mucosa, or lymph node associated with pulmonary radiologic findings in keeping with KS (n = 3) or pulmonary KS found at autopsy (n = 1) without evidence of other pulmonary disease or infection (n = 4); temporal association between initiation of HAART and worsening of pulmonary signs and symptoms associated with concomitant worsening of cutaneous and/or mucosal KS lesions (n = 4); favorable response to HAART (> 1 log10 reduction in HIV RNA) with or without an associated 50% CD4 increase to
[in this window]
[in a new window]
The HAART regimens consisted of two nucleosides combined with a nonnucleoside reverse transcriptase inhibitor or a protease inhibitor. HIV RNA and CD4+ cell count were measured before initiation of antiretroviral therapy in all four cases and at the time of diagnosis of KS-associated immune reconstitution syndrome in three cases. The immune response to HAART was reflected by a reduction in HIV RNA log10 of 2.6, 3.0, and 3.3 in the three cases in which comparison was available. One patient had no increase in CD4 cell count, and two patients had increases from 90 and 40 cells/µL to 160 and 120 cells/µL, respectively. In the fourth case, HIV RNA and CD4 cell count after initiation of HAART were not measured because the patient died after rapid deterioration of his clinical condition. The mean interval from the start of HAART and establishment of the diagnosis of KS-associated immune reconstitution syndrome was 9.2 weeks (range, 1–22 weeks).
The images of the four patients were reviewed retrospectively. Two experienced radiologists jointly interpreted the chest radiographs and thoracic CT scans and reached a decision by consensus. The reviewers were aware that all patients had the diagnosis of KS-associated immune reconstitution syndrome. Images reviewed in the study included one chest radiograph per patient obtained before initiation of HAART, except in one case, in which the only radiographs obtained before HAART were acquired during an episode of P. jiroveci (Pneumocystis carinii) pneumonia and therefore were excluded; one radiograph and chest CT scan per patient obtained during initial symptoms of KS-associated immune reconstitution syndrome; and one follow-up radiograph and chest CT scan per patient obtained during immune reconstitution syndrome.
The CT scans were obtained with a 4-MDCT scanner (LightSpeed Plus, GE Healthcare). The scans were acquired at end inspiration from the apex of the lung to the diaphragm at 120 kV and 210 mA and reconstructed to slice thicknesses of 1.0 mm (n = 2), 1.25 mm (n = 2), and 5 mm (n = 4). In one case, follow-up CT was performed 105 days after the initial CT scan, after the patient underwent chemotherapy. The other patients did not undergo chemotherapy and underwent follow-up CT 14–20 days after the initial CT examination (mean, 16 days). A total of eight chest radiographs obtained during KS-associated immune reconstitution syndrome were reviewed, two per patient. These radiographs were acquired 1–72 days after the CT scans (mean interval, 10.5 ± 24.9 days).
Chest radiographs were reviewed for hilar and mediastinal lymphadenopathy, reticular and reticulonodular opacities, Kerley B lines, parenchymal consolidation, pulmonary nodules, and pleural effusions. The chest CT scans were reviewed for the presence or absence and anatomic distribution of ground-glass opacities, consolidation, nodules, interlobular septal thickening, peribronchovascular thickening, irregular linear opacities (reticulation), the halo sign, emphysema, pulmonary cysts, mediastinal, hilar, and axillary lymphadenopathy, pleural nodularity, and pleural effusion. These findings were defined according to the Fleischner Society nomenclature [23].
The radiologic findings were classified by one chest radiologist as mild, moderate, or severe on the basis of visual assessment of extent and severity to characterize progression, stability, or regression of disease. The extent of consolidation was classified as involving less than 5%, 5–25%, 26–50%, or more than 50% of the pulmonary parenchyma. These scores were based on visual assessment of the percentage of involvement of each lobe (the lingula was considered a separate lobe) as approximately 0, 1/4, 2/4, 3/4, or 4/4. Each lower lobe was considered to represent 20% of the pulmonary parenchyma; the upper lobes, right middle lobe, and lingula were considered to represent 15% each. The overall pulmonary extent of consolidation represented the sum of the lobar extent. Pulmonary nodules were classified as having well-defined or ill-defined margins. Axillary, mediastinal, or hilar lymphadenopathy was considered present when the lymph node short-axis diameter was greater than 10 mm. When present, lymphadenopathy was classified as mild when the nodal diameter was less than 20 mm, moderate when the diameter was between 20 and 30 mm, and severe when the diameter was greater than 30 mm.
The anatomic distribution of the abnormalities on chest radiographs was classified as predominantly in the upper, middle, or lower lung zone and perihilar, peripheral, or random. On CT the anatomic distribution was classified as follows: central when there was a predominance of abnormalities in the inner third of the lung, peripheral when there was a predominance of abnormalities in the outer third of the lung, and random when there was no central or peripheral predominance. Zonal predominance was assessed as being upper, middle, lower, or random. Upper lung zone predominance was considered present when most of the abnormalities were above the level of the tracheal carina, middle zone predominance when between the carina and the inferior pulmonary veins, lower zone predominance when most of the abnormalities were below this level, and random when there was no zonal predominance. The findings also were classified as unilateral or bilateral. The images were reviewed with lung (width, 1,500 H; level, –600 H) and mediastinal (width, 350 H; level, 35–40 H) windows.
Results
All patients had abnormal chest radiographic and CT findings at presentation and follow-up of KS-associated immune reconstitution syndrome (Tables 2 and 3). Chest radiographs showed reticular opacities in one of the four patients, ill-defined nodules associated with reticular opacities in three, and septal (Kerley B) lines in three patients (Figs. 1A, 1B, 1C and 2A, 2B, 2C, 2D). Consolidation was found on the radiographs of two patients during the onset of KS-associated immune reconstitution syndrome and in three patients on the follow-up radiograph (Figs. 1A, 1B, 1C and 2A, 2B, 2C, 2D). The radiographic findings were predominantly located in a perihilar distribution in three of the four patients and were diffuse in the other patient. The abnormalities involved mainly the middle and lower lung zones in three patients and showed no predominance in the other patient. Pleural effusion was present in two patients on the radiograph during the onset of immune reconstitution syndrome and in three patients on the follow-up radiograph. Mediastinal and hilar lymphadenopathy were not detected in any of the four subjects.
[in this window]
[in a new window]
[in this window]
[in a new window]
View larger version (142K):
[in this window]
[in a new window]
[as a PowerPoint slide]
View larger version (142K):
[in this window]
[in a new window]
[as a PowerPoint slide]
View larger version (144K):
[in this window]
[in a new window]
[as a PowerPoint slide]
View larger version (154K):
[in this window]
[in a new window]
[as a PowerPoint slide]
View larger version (145K):
[in this window]
[in a new window]
[as a PowerPoint slide]
View larger version (151K):
[in this window]
[in a new window]
[as a PowerPoint slide]
View larger version (145K):
[in this window]
[in a new window]
[as a PowerPoint slide]
The chest radiograph obtained before initiation of HAART was compared with a radiograph obtained during the onset of KS-associated immune reconstitution syndrome in three of the four cases. The latter radiographs showed mild progression of the abnormalities in one patient and marked progression in the other two patients (Table 2). Comparison between the chest radiograph obtained during the onset of KS-associated immune reconstitution syndrome and the follow-up radiograph was performed in all four patients. Rapid progression of the abnormalities was found in the three patients who did not receive chemotherapy. Subtle improvement was found in the patient who received chemotherapy.
Irregular and ill-defined pulmonary nodules measuring 3–27 mm in diameter (Figs. 2A, 2B, 2C, 2D and 3A, 3B, 3C, 3D) were found on CT scans of all patients. The halo sign, consolidation, and ground-glass opacities were present in three patients (Figs. 2A, 2B, 2C, 2D, 3A, 3B, 3C, 3D, 4A, 4B, 4C, 4D). When present, nodules, consolidations, and ground-glass opacities were bilateral in all patients. These findings had lower-zone predominance in two patients, middle zone predominance in one patient, and no zonal predominance in one patient. The anatomic distribution was predominantly central peribronchovascular in all patients. The consolidations in the three patients who did not undergo chemotherapy increased in extent during the 14- to 20-day follow-up period. The consolidations involved less than 5% of the parenchyma on the initial CT scans of all three patients, 26–50% in two patients, and more than 50% in one patient at follow-up evaluation.
View larger version (154K):
[in this window]
[in a new window]
[as a PowerPoint slide]
View larger version (161K):
[in this window]
[in a new window]
[as a PowerPoint slide]
View larger version (139K):
[in this window]
[in a new window]
[as a PowerPoint slide]
View larger version (138K):
[in this window]
[in a new window]
[as a PowerPoint slide]
View larger version (136K):
[in this window]
[in a new window]
[as a PowerPoint slide]
View larger version (142K):
[in this window]
[in a new window]
[as a PowerPoint slide]
View larger version (142K):
[in this window]
[in a new window]
[as a PowerPoint slide]
View larger version (144K):
[in this window]
[in a new window]
[as a PowerPoint slide]
Peribronchovascular and interlobular septal thickening was found in all subjects (Figs. 2A, 2B, 2C, 2D, 3A, 3B, 3C, 3D, 4A, 4B, 4C, 4D). Pulmonary cysts and paraseptal and centrilobular emphysema were found in one patient each. Pleural nodularity was present in three patients (Fig. 2A, 2B, 2C, 2D). Pleural effusion was present in two of the subjects on initial CT and in three on follow-up CT (Figs. 2A, 2B, 2C, 2D, 3A, 3B, 3C, 3D, 4A, 4B, 4C, 4D). Mediastinal and axillary lymphadenopathy were found in three and two of the patients, respectively. When present, lymphadenopathy had high attenuation (38–95 H; mean, 68.8 ± 20.6 H) on CT scans obtained after contrast administration.
Discussion
KS is the most common tumor among patients with HIV infection, occurring predominantly among homosexual or bisexual men [24]. KS is associated with human herpesvirus 8 infection [24, 25]. Previous studies [1, 13, 24, 26–28] have shown that use of HAART has resulted in substantial diminution in the incidence, morbidity, and mortality of KS. Furthermore, HAART alone can lead to stabilization and regression of KS, often eliminating the need for chemotherapy and radiation therapy in the treatment of many patients with HIV infection [14] and prolonging remission among patients with a complete response [15]. Holkova et al. [13] analyzed the cases of 37 consecutively registered patients with pulmonary KS and HIV infection and found that 90% of the patients in the pre-HAART period died of progressive pulmonary KS, a finding in agreement with other published data. In the post-HAART period, 47% of patients died of progressive pulmonary KS, a significant improvement in overall survival rate.
Although in most cases of KS clinical improvement occurs after HAART, KS-associated immune reconstitution syndrome after initiation of HAART has been reported [3, 4, 6–8, 16–22]. The onset of the syndrome is probably related to an increased immune response to human herpesvirus 8 characterized by inflammation, edema, and worsening of KS lesions. To our knowledge, in 1997 Weir and Wansbrough-Jones [20] described the first case of KS flare. The patient experienced airway obstruction as a result of worsening KS of the larynx 4 weeks after the start of HAART despite improvement in the CD4 cell count and effective HIV viral suppression. Connick et al. [7] later reported one case of KS-associated immune reconstitution syndrome in a patient with cutaneous lesions consistent with KS. During the second week of HAART, face and neck swelling, new cutaneous lesions, and cervical lymphadenopathy developed, and excisional biopsy proved the diagnosis of KS. The patient's condition improved after chemotherapy. Rodríguez-Rosado et al. [22] also reported a case of cutaneous KS that exhibited an explosive increase in number and size of the lesions and the development of lymphedema after the initiation of HAART. Shelburne et al. [4] described one case in which recovery of the immune system with HAART resulted in worsening of both sarcoidosis and cutaneous KS. Bower et al. [8] reported a 6.6% incidence of KS-associated immune reconstitution syndrome among 150 patients with KS who started HAART. In these cases, none of the patients with immune reconstitution syndrome had pulmonary KS. Similarly, Leidner and Aboulafia [6] reported nine cases of cutaneous, mucocutaneous, or lymphatic KS-associated immune reconstitution syndrome.
Although most previous reports describe cases of KS-associated immune reconstitution syndrome with no visceral involvement, there are reports [4, 16, 18, 19] of pulmonary KS-associated immune reconstitution syndrome (Table 1). The data on the radiologic findings in these patients, however, are limited. Crane et al. [16] described a patient with a fatal inflammatory response to pulmonary KS after initiation of HAART. The chest radiograph was described as having a prominent interstitium and small pleural effusion. The chest CT findings confirmed the radiographic findings and showed splenomegaly and lymphadenopathy. Sánchez Ayuso et al. [18] described a case of fatal pulmonary KS-associated immune reconstitution syndrome. In that case, CT of the thorax showed mediastinal and bilateral axillary lymphadenopathy, paraseptal emphysema, consolidation, ill-defined nodular opacities, ground-glass opacities, and interlobular septal thickening with a predominant central distribution. Ball [19] reported a case of pulmonary KS that worsened after initiation of HAART. There was also associated worsening of herpes simplex and nontuberculous mycobacterial infection. CT showed an increase in the number and size of pulmonary nodules and adjacent ground-glass opacities. The patient's condition improved after chemotherapy.
The interval between initiation of HAART and development of immune reconstitution syndrome is variable [4, 9, 10, 28, 29]. Hirsch et al. [29] found the interval varied from less than 1 week to several months. In the series reported by Ratnam et al. [10], seven (14%) of 51 episodes of immune reconstitution syndrome in 44 patients occurred 24 weeks after HAART. Shelburne et al. [4] reviewed three cases of KS-associated immune reconstitution syndrome in which the intervals between HAART and symptoms were 3.6, 8.6, and 42.8 weeks. In our series, the interval between initiation of HAART and onset of pulmonary KS-associated immune reconstitution syndrome ranged from 1 to 22 weeks.
The radiographic and CT findings of AIDS-related KS are well described in the literature, but to our knowledge, except for a few case reports [16, 18, 19] mentioning radiologic findings, no study has been focused on the imaging features of pulmonary KS-associated immune reconstitution syndrome. The well-known characteristic radiographic finding of AIDS-related KS is the presence of bilateral interstitial or alveolar opacities, often associated with poorly defined nodular opacities [30–33]. These findings are frequently accompanied by pleural effusions, occurring in as many as 67% of patients [30–34]. Lymphadenopathy is present in nearly 10% of cases [30, 31, 34]. Peripheral linear opacities (Kerley B lines) also have been reported [31]. In a few patients with minimal involvement of the lung, the chest radiographic findings may be normal [30, 31, 34].
The typical CT findings of AIDS-related KS include bilateral ill-defined nodular opacities in a peribronchovascular distribution, which are also described as flame-shaped lesions [35, 36]. Areas of ground-glass attenuation surrounding one or more nodules, the so-called halo sign, have also been described [37, 38]. Nodules usually measure 1–2 cm in diameter and tend to coalesce [33, 34]. Areas of consolidation and ground-glass opacities separated from nodules also may be seen [37, 39]. Previous studies have shown that pleural effusions are present in 35–60% and lymphadenopathy (axillary, mediastinal, or hilar) in 33–53% of patients [35, 37, 39, 40]. The enlarged lymph nodes usually measure less than 20 mm in diameter [35]. Other common findings include peribronchovascular and interlobular septal thickening and fissural nodularity [37]. Larger masses and cavities may be seen but are less common [39]. Chest wall disease involving the sternum, ribs, thoracic spine, and subcutaneous tissue has been described [40].
The findings in our study concur with the radiologic findings in previous case reports of KS-associated immune reconstitution syndrome [16, 18, 19]. We found that patients with this syndrome have radiographic and CT findings similar to those of patients with AIDS-related KS without immune reconstitution syndrome. However, the incidence of pleural effusions and lymphadenopathy was higher than described for patients with AIDS-related KS without immune reconstitution syndrome. A notable difference was found in the follow-up of these patients. All patients in our series, except one who received chemotherapy, had marked progression of pulmonary abnormalities soon (mean, 16 days; range, 14–20 days) after the onset of immune reconstitution syndrome.
Therefore, rapid progression of pulmonary abnormalities on chest radiographs and CT scans in association with acute worsening of pulmonary signs and symptoms in patients who have recently started HAART does not necessarily indicate a diagnosis of infection. When radiologic findings are compatible with pulmonary KS, the possibility of KS-associated immune reconstitution syndrome should be considered, particularly in association with cutaneous or mucosal lesions compatible with KS. The diagnosis of this syndrome requires exclusion of other conditions that can cause similar findings, particularly P. jiroveci and community-acquired pneumonia. Other differential diagnoses include drug toxicity and progression of disease not related to initiation of HAART. In the latter case, there is no temporal relation between initiation of antiretroviral therapy and the onset of the symptoms, and the viral load and CD4 cell count usually do not respond to HAART [14, 41].
As shown in previous reports of pulmonary KS-associated immune reconstitution syndrome [16, 18], bronchoscopy may be unrevealing, as it was in all four of our patients, including the one whose autopsy showed extensive pulmonary KS. The main value of negative bronchoscopic findings is in excluding infection as a cause of clinical deterioration. Surgical lung biopsy can be performed if histologic diagnosis is necessary. However, because patients with immune reconstitution syndrome may have severe clinical deterioration, the risk-to-benefit ratio of open lung biopsy needs to be considered for each patient. If enough evidence of disseminated KS is proved by the presence of skin or mucosal lesions and if radiologic features are in keeping with pulmonary KS and infectious diseases have been excluded, surgical lung biopsy can be avoided. This scenario occurred in one of our cases: The patient received chemotherapy for disseminated KS without histologic proof of pulmonary KS, and all KS lesions, including the pulmonary lesions, were ameliorated.
The anecdotal nature of previous reports precludes firm recommendations regarding therapy. There are no guidelines regarding the continuation or interruption of HAART. Antiretroviral therapy usually is discontinued when inflammatory reactions are considered life threatening [29], as in the case of pulmonary KS-associated immune reconstitution syndrome. In the study performed by Bower et al. [8], despite initial deterioration, the condition of 10 patients with KS-associated immune reconstitution syndrome improved with continuation of HAART. None of those patients, however, had pulmonary involvement. The use of antiinflammatory agents, especially corticosteroids, is usually recommended for other immune reconstitution syndromes in severe cases.
Although the prognosis of KS-associated immune reconstitution syndrome is usually good [8], pulmonary involvement in patients with this syndrome seems to indicate a poor outcome, as shown in our cases and in previous reports [16–18]. The institution of chemotherapy can be beneficial to patients with pulmonary KS-associated immune reconstitution syndrome. In our small series the one patient who survived was the only one who received chemotherapy. The other three patients did not receive this treatment because of rapid clinical deterioration. The few cases reported in the literature had similar outcome [16–19]. The limitations of our study included its retrospective character, the small number of patients, and the lack of histologic and cytologic confirmation of pulmonary KS in three of the four patients.
In conclusion, although HAART is the mainstay of management of KS, a few patients experience worsening of KS during the first several months of therapy, and radiologists must be aware of the radiologic manifestations of pulmonary KS-associated immune reconstitution syndrome. The most common chest radiographic findings of this syndrome include ill-defined nodules, reticular and reticulonodular opacities, and consolidation with a predominantly perihilar distribution. Kerley B lines and pleural effusions also are common findings. The CT findings are ill-defined pulmonary nodules and peribronchovascular and interlobular septal thickening. Other common findings include the halo sign, consolidation, ground-glass opacities, nodular pleural thickening, lymphadenopathy, and pleural effusion. These abnormalities have a predominantly peribronchovascular distribution and may show marked progression in a short time.
References
International Collaboration on HIV and Cancer. Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. J Natl Cancer Inst2000; 92:1823 –1830[Abstract/Free Full Text] Palella FJ Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med 1998;338 : 853–860[Abstract/Free Full Text] Crum-Cianflone NF. Immune reconstitution inflammatory syndromes: what's new? AIDS Read 2006;16 : 199–206, 213, 216–217[Medline] Shelburne SA, Hamill RJ, Rodriguez-Barradas MC, et al. Immune reconstitution inflammatory syndrome: emergence of a unique syndrome during highly active antiretroviral therapy. Medicine (Baltimore) 2002; 81:213 –227[CrossRef][Medline] Robertson J, Meier M, Wall J, Ying J, Fichtenbaum CJ. Immune reconstitution syndrome in HIV: validating a case definition and identifying clinical predictors in persons initiating antiretroviral therapy. Clin Infect Dis 2006;42 :1639 –1646[CrossRef][Medline] Leidner RS, Aboulafia DM. Recrudescent Kaposi's sarcoma after initiation of HAART: a manifestation of immune reconstitution syndrome. AIDS Patient Care STDS 2005;19 : 635–644[CrossRef][Medline] Connick E, Kane MA, White IE, Ryder J, Campbell TB. Immune reconstitution inflammatory syndrome associated with Kaposi sarcoma during potent antiretroviral therapy. Clin Infect Dis2004; 39:1852 –1855[CrossRef][Medline] Bower M, Nelson M, Young AM, et al. Immune reconstitution inflammatory syndrome associated with Kaposi's sarcoma. J Clin Oncol 2005; 23:5224 –5228[Abstract/Free Full Text] Phillips P, Kwiatkowski MB, Copland M, Craib K, Montaner J. Mycobacterial lymphadenitis associated with the initiation of combination antiretroviral therapy. J Acquir Immune Defic Syndr Hum Retrovirol 1999; 20:122 –128[Medline] Ratnam I, Chiu C, Kandala NB, Easterbrook PJ. Incidence and risk factors for immune reconstitution inflammatory syndrome in an ethnically diverse HIV type 1-infected cohort. Clin Infect Dis2006; 42:418 –427[CrossRef][Medline] Phillips P, Bonner S, Gataric N, et al. Nontuberculous mycobacterial immune reconstitution syndrome in HIV-infected patients: spectrum of disease and long-term follow-up. Clin Infect Dis 2005; 41:1483 –1497[CrossRef][Medline] Di Lorenzo G, Konstantinopoulos PA, Pantanowitz L, Di Trolio R, De Placido S, Dezube BJ. Management of AIDS-related Kaposi's sarcoma. Lancet Oncol 2007;8 : 167–176[CrossRef][Medline] Holkova B, Takeshita K, Cheng DM, et al. Effect of highly active antiretroviral therapy on survival in patients with AIDS-associated pulmonary Kaposi's sarcoma treated with chemotherapy. J Clin Oncol 2001; 19:3848 –3851[Abstract/Free Full Text] Cattelan AM, Calabro ML, Gasperini P, et al. Acquired immunodeficiency syndrome-related Kaposi's sarcoma regression after highly active antiretroviral therapy: biologic correlates of clinical outcome. J Natl Cancer Inst Monogr 2001:44 –49[Abstract/Free Full Text] Cattelan AM, Calabro ML, De Rossi A, et al. Long-term clinical outcome of AIDS-related Kaposi's sarcoma during highly active antiretroviral therapy. Int J Oncol 2005;27 : 779–785[Medline] Crane HM, Deubner H, Huang JC, Swanson PE, Harrington RD. Fatal Kaposi's sarcoma–associated immune reconstitution following HAART initiation. Int J STD AIDS 2005;16 : 80–83[Abstract/Free Full Text] Harindra V, Foley E. Highly active antiretroviral therapy. Lancet 1998; 351:1058[Medline] Sánchez Ayuso FJ, Moreno Mendana JM, del Palacio Lopez-Medel A. Kaposi sarcoma in a patient with AIDS in the syndrome of immune reconstitution [in Spanish]. An Med Interna2006; 23:95 –96[Medline] Ball SC. Immune reconstitution in several forms. AIDS Read 2005; 15:17 –20[Medline] Weir A, Wansbrough-Jones M. Mucosal Kaposi's sarcoma following protease inhibitor therapy in an HIV-infected patient. AIDS 1997; 11:1895 –1896[Medline] Chan DJ. Kaposi's sarcoma–associated immune reconstitution following highly active antiretroviral treatment initiation. Int J STD AIDS 2006; 17:72[CrossRef][Medline] Rodríguez-Rosado R, Soriano V, Dona C, González-Lahoz J. Opportunistic infections shortly after beginning highly active antiretroviral therapy. Antivir Ther1998; 3:229 –231[Medline] Austin JH, Muller NL, Friedman PJ, et al. Glossary of terms for CT of the lungs: recommendations of the Nomenclature Committee of the Fleischner Society. Radiology 1996;200 : 327–331[Free Full Text] Mbulaiteye SM, Parkin DM, Rabkin CS. Epidemiology of AIDS-related malignancies: an international perspective. Hematol Oncol Clin North Am 2003; 17:673 –696[CrossRef][Medline] Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 1994; 266:1865 –1869[Abstract/Free Full Text] Jacobson LP, Yamashita TE, Detels R, et al. Impact of potent antiretroviral therapy on the incidence of Kaposi's sarcoma and non-Hodgkin's lymphomas among HIV-1-infected individuals: multicenter AIDS cohort study. J Acquir Immune Defic Syndr 1999;21 [suppl 1]:S34 –S41[Medline] Grulich AE. Update: cancer risk in persons with HIV/AIDS in the era of combination antiretroviral therapy. AIDS Read2000; 10:341 –346[Medline] Portsmouth S, Stebbing J, Gill J, et al. A comparison of regimens based on non-nucleoside reverse transcriptase inhibitors or protease inhibitors in preventing Kaposi's sarcoma. AIDS2003; 17:F17 –F22[CrossRef][Medline] Hirsch HH, Kaufmann G, Sendi P, Battegay M. Immune reconstitution in HIV-infected patients. Clin Infect Dis2004; 38:1159 –1166[CrossRef][Medline] McLoud TC, Naidich DP. Thoracic disease in the immunocompromised patient. Radiol Clin North Am 1992;30 : 525–554[Medline] Davis SD, Henschke CI, Chamides BK, Westcott JL. Intrathoracic Kaposi sarcoma in AIDS patients: radiographic-pathologic correlation. Radiology 1987;163 : 495–500[Abstract/Free Full Text]
Let me see...
radiographia.ru
ОГРОМНОЕ СПАСИБО!!!
Всегда рад! Единственное сожалею, что у меня нет материалов на русском языке...
Let me see...
radiographia.ru